(the Registrar calls it Chem 124)
Freshman
Organic Chemistry
Fall 2008
If you are viewing this page through Yale's ClassesV2 window, you may prefer to access it directly so as to have the whole screen available. For Direct Access log out of ClassesV2 and go to https://webspace.yale.edu/chem125/
Material for First Quarter of the Fall Semester 2008
Material for Second Quarter of the Fall Semester 2008
Material for Third Quarter of the Fall Semester 2008
Do read this
Lecture 1 (9/3/08) zipped or (raw)
(The BIG Question ; The Royal Society ; Pepys as a model Chem 125 student; Force Laws and Springs)
Samuel Pepys and Isaac Newton - How might they do in Chem 125?
You may work in groups of up to 6 to prepare a group hard copy or e-mail submission (with the names of all participants on it).
Lecture 2 (9/5/08) zipped or (raw)(A Chemical Force Law? ; Lewis's Electron Octet; equilibrium vs. resonance; acetic acid geometry & energy; Lore)
note Problems for Wednesday on frame 40 (Lewis structure for H/C/N in that order, H/C/H/O in any order)
( Newton's Chemistry & the Business of Experimental Philosophy)
Lecture 3 (9/8/08) zipped or (raw)
( O2/O3 4-D plotting; charge distribution; Earnshaw; Resonance Lore ; Earnshaw and Faraday's Lines of Force ; Plum Pudding Atoms ; Was Coulomb Wrong?)
Dealing
with Graphs - Lesson 1
Drill
on multidimensional plotting
(you
don't need to turn the
problems in, but you should know how to do them)
Functional Group Table
An
organic chemistry
course is supposed to have a lot of memorizing. We try to
keep
this to a minimum, but the minimum isn't nothing. For the first hour exam you will
be responsible for memorizing the names of 17 "functional groups"
and how to recognize and draw simple "constitutional" structures for
them. These functional groups are identified in red in a
table
that includes 16 additional functional groups that we'll probably
encounter this semester. You can access the table in three
formats: as a web
page ; as a pdf
file ; as an downloadable Excel
Spreadsheet.
I find that sorting a spreadsheet different ways and stepping
through it is an effective tool for memorization - that's how I learn
your names. Good luck.
The first subject
of the course is
how we know about the existence of atoms, molecules, bonds and the
role of authority in science. To begin we will contrast two erroneous
treatments of atomic and molecular structure at the turn of the last
century. One of them is by two great scientists G. N. Lewis (Lewis
dot structures) and J. J. Thomson (discoverer of the electron). The
other is by "clairvoyant" frauds.
You may enjoy
reading some of the
web material in preparation for the lectures:
Lecture 4 (9/10/08) zipped or (raw)
(Clairvoyant "Science" ; Powers of 105 ; Measuring the very small - Franklin; Feeling atoms SPM)
[Seeing
Atoms with Clairvoyance]
relevant, but not assigned reading this year
If you're
skeptical that Crookes really believed in psychic pheomena, click here
(also not assigned, just for the curious)
SPM
web page
Friday's
lecture will begin X-Ray
Diffraction
It will be VERY helpful to read ahead on the x-ray page
Lecture 5 (9/12/08) zipped or (raw)
( light and seeing by wave
interference, x-ray diffraction, molecular and lattice
diffraction )
Diffraction examples from lecture demonstration
Lecture 6 (9/15/08) zipped or (raw)
(understanding molecular and lattice
diffraction, Franklin's DNA; e-density maps, difference
maps and bonding)
NOTE: for cross-platform compatibility audio and video files have been removed from the Lecture 6 links above.
To try for the whole package, click here.
Leslie
Leiserowitz on his Triene (Transcript 16K)
Leiserowitz Quicktime Movie
(160 x 120, 5.6 Meg) or (240
x 180, 10.3 Meg)
Dunitz Quicktime Movie (160 x 120, 6.2 MB) or (240 x 180, 11.2 MB)
The Second Great Question
DO READ THIS: Quantum Mechanics (Sections I-V on 1st Exam)
Lecture 7 (9/19/07) zipped (includes sound file) or (raw - without sound, zipped without sound)
(Pathological Bonding; Schroedinger's Invention; Weird Kinetic Energy; The Jeopardy Method)
Erwin Meets Goldilocks (problem set due Monday, Sept. 22 - PDF document)
|
Filip will demonstrate the use of Erwin Meets Goldilocks at the Thursday discussion section. This program will help you develop a quantum mechanical intuition. You will be doing the relevant problem set in self-selected groups. Be sure that at least one representative from your group (or someone else who is willing to help you) attends the demonstration session. Bring your laptop to the session. It would help if you download the Applet ahead of time. |  thanks to Yoonjoo Lee |
Lecture 8 (9/19/08) zipped and Raw
(1D Quantum - Erwin meets Goldilocks; Nodes & Quantization; Psi-squared & Structure; Vibration)
Lecture 9 (9/22/08) zipped or Raw or Stripped of Troublesome Juggling Video
(Double Minima - bonding and tunneling; Chladni - nodes in 2D; H-like atom wave functions; rho for r, orbital shapes, Parts of 2p; Dauger's Atom-in-Box)
Chladni Figure Short Movie (Quicktime - 3MB)
4 Chladni Figures (Quicktime - 9.5 MB)
Quantum Mechanics V: H-like Atoms & problem set
Problems
for Wednesday, September 24 :
2)Why are the first two cells [(0,0) (1,0)] in Chladni's tables vacant?
3) Compare 1s of H with 2s of C+5 in Energy
4) Do the six Atomic Orbital problems HERE (also for Atom-in-a-Box Download)
Lecture 10 (9/24/08) zipped or Raw
(Atom-in-Box; r2 weighting; scaling size and energy; hybridization for orientation and shape; The Problem With Orbitals)
If you
are have difficulty with why r2 is
sometimes used to multiply ψ2 in
discussing electron distribution,
you might try these optional
problems from a previous year and discuss them with friends
or TAs.
There will be other review sessions on Thursday 7-10, 116 WLH (Filip and Peer Tutors)
You will have a full hour to write Friday's exam. You may start at 10:30 and end at 11:30 in the normal lecture room (160 SCL)
or if it's important to get somewhere else by 11:30, you may choose the early option: start at 10:15 and end at 11:15.
For the "early option" come to Room 111 SCL (at the other end of the hall from 160).
You may be wondering what kind of questions might appear on exams in this course. (Our first exam will occur on Friday, Sept. 26 The following PDF file contains a number of questions based on topics we have been discussing. These would be fair game. It might be useful to look them over as we go along.
Sample questions for first hour exam
Sample Quantum Mechanics Questions (PDF)
Sample questions on Early Topics (pdf file)
First Hour Exam 2008 (pdf) First Hour Exam 2008 Answer Key(pdf)
| The first exam papers will be returned in class on Monday, 9/29. The class average was very good (80.0) with 1/3 of the scores greater than 85 and 2/3 of the scores greater than 76. Congratulations, you seem to be learning a lot! You can check your own points, both by question and total, using Post'em on ClassesV2. If you have questions about the grading, consult the answer key (above) first, and then speak to the grader of the relevant question (1-5, Schley; 6-8, McBride; 9-11, Kolundzic). The "letter grades" posted there are of course almost insignificant, because they represent such a small, preliminary portion of the grading for the semester. But the course content is cumulative, so it can be helpful to see where you stand thus far, and whether it is important to be sure you're not falling behind. There is plenty of help available from the teaching staff. 5/6 of the semester scoring is still to come, so it's way too early either to despair or to become self-satisfied. The plot to the right from year before last shows that the first exam score was not a great predictor of semester total. There is some correlation, but it is easy by working (or not) to move up (or down) by a letter grade. | 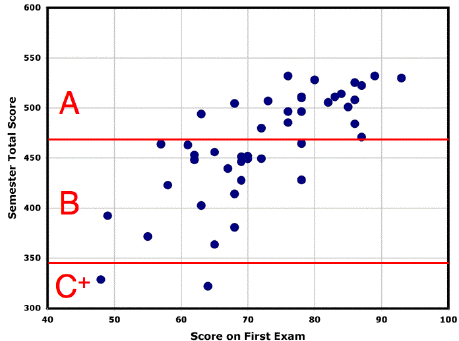 |
Material for the second quarter of the Fall Semester 2008
Lecture 11 - 9/29/08 zipped or Raw
(2-electrons,
Zeff,
SCF,
"Correlation Energy", Energy Magnitudes; Orbital
Shapes,
"Plum-Pudding" molecular orbitals)
Quantum
Mechanics VI-VII
(orbitals for several electrons, hybridization)
(Orbital Shapes, "Plum-Pudding" molecular orbitals; Pairwise LCAO-MO, Bonding in H2)
Quantum
Mechanics VIII-IX
(molecular orbitals; HOMOs and LUMOs for functional groups)
Note that preliminary versions of lecture powerpoints include material that will probably not be discussed until the following lecture. This will allow people who will be assigned the related wikis to have more time to prepare.
H-H Bonding
C-C
Overlap and Hybridization
Lecture 13 - 10/3/08
(PowerPoint) or Raw
(C-C Overlap, Overlap
& Energy, Newton's Grail; Energy-Match, H2 vs. HF)
Lecture 14 - 10/6/07
(PowerPoint) or Raw
(XH3:
IR, ESR, CF3; Goals)
XH3 (contact with reality)
Lecture 15 - 10/8/08
(PowerPoint) or Raw
(Pathological Bonds; BH3 and
local bonds; Which
mixings are important?; Acid-Base Theory; What makes HOMO high, LUMO
low?)
BH3,
an Example of an MO Computation & an Apology for Local Bonds
Note: the pictures on this page are fun to look at, but
don't
worry too much about the verbal content,
unless you can't sleep because we use local bond analysis instead of
the MOs that computers find.
MO
Interactions, which ones count? (HOMO/LUMO)
Lecture 16 - 10/10/08
(PowerPoint) or Raw
(What makes HOMO high, LUMO
low? B2H6; HF LUMO; Acid-Base
- SN2 - E2 LUMO Analogies)
Orbital Shape
| | |
| | |
| |
Lecture 17 - 10/13/08
(PowerPoint) or Raw
(Acid-Base
- SN2 - E2 LUMO Analogies; Make & Break; N2H4
Synthesis; Four
Functional Groups: carbonyl)
LUMO Analogies (elimination
from C2H5X)
Lecture 18 - 10/15/08 (PowerPoint) or Raw
(Four Functional Groups, cont.: amide, carboxylic acid, C-metal bond, How Did They Know? Chemical Cousins)
Intramolecular HOMO/LUMO mixing and
"Resonance"
3-Center
"Electron-Deficient" "Y" Bonds
[ B2H6
& (CH3Li)4
•4 OMe2]
See Wiki for Functional Group Analysis Problem. All topics have not been chosen/assigned
History
(how humans actually
learned about organic chemistry)
Your Genealogy in Organic Structure Theory
NOTE: Lecture 19 will be covered on the Third Exam, not on Monday's Second Exam. Wikis due next Tuesday.
Preliminary Lecture 19 - 10/17/08 (PowerPoint) or Raw
(Precursors of Modern Chemistry- Alchemy, Scheele's Acids; Oxygen; Lavoisier's Revolution: Nomenclature, Traité, Analysis, Elements, Measuring Gases, Radicals & Language, Measuring Heat)
Review Sessions for Monday's Exam
Prof. McBride is will lead another session Thursday 8:30-10:30 PM in WLH 116, displacing Filip Kolundzic's session.
Filip will be contacting you by e-mail to find what time he could offer a review session to accommodate those who cannot come on Thursday evening.
The Peer Tutors will have their regularly scheduled help session on Sunday evening.
Sample Problems for Monday's Exam
| 2nd Hour Exam 2007 (PDF) | 2nd Hour Exam 2007 Answers (PDF) |
| 2nd Hour Exam 2006 (PDF) | 2nd Hour Exam 2006 Answers (PDF) |
| 2nd Hour Exam 2005 (PDF) | 2nd Hour Exam 2005 Answers |
| | |
| 2nd Hour Exam 2003 (pdf) | 2nd Hour Exam 2003 Answer Key (pdf) |
| 2nd Hour Exam 2002 (Word)... (PDF) | Answers to 2002 2nd Exam (Word)... (PDF) |
| 2nd Hour Exam 2000 (Word) | Answers to 2000 2nd Exam |
| 2nd Hour Exam 1999 (web page) | (answers at end) |
| 2nd Hour Exam from 1998 (Word) | Answers to 1998 2nd Exam |
The exam will take place at 10:30 AM (normal lecture time) in SCL 160 (normal lecture room). It will be budgeted for 50 minutes, but 60 minutes will be allowed for writing exam papers. To accommodate students who must leave for classes beginning at 11:35 (or who just wish to get it over with), the exam may be taken early from 10:15-11:15 in SCL 111 (southeast corner of SCL - at other end of the hall from 160).
Second Hour Exam 2008 (pdf) Second Hour Exam 2008 Answer Key(pdf)
Material for 3rd Quarter of First Semester 2008
Prerevolutionary contributions - Scheele
Lecture 20 - 10/22/08
(zipped) or Raw
(
Lavoisier: Analysis & Bookkeeping; Dalton: Atoms &
Proportions;
Berzelius: Symbols & Analysis; Gay-Lussac Volumes)
Lavoisier & the Chemical Revolution: Analysis
(14 Questions for Friday indicated by * below. You may work in small groups)
Portrait (we discussed the problem re. Fig. 10 in class, you need not turn it in)Preface (1789)
Elements & Oxidation *2
How to determine Gas Density
Calorimeter *4
Combustion of Phosphorus and Carbon *4
Quantitative Chemical "Formulae" *4
Lecture 21 - 10/24/08
(zipped) or Raw
(Mitscherlich As/P ratio; Berzelius: Analysis, Electrolysis
& Dualism; Woehler & Urea; Isomerism; Liebig Analysis)
Combustion Analysis Lavoisier/Prout/Liebig/Dumas (1788-1841)
Note: problems for Wednesday (on web pagess with red years 1828 and 1832 below)
Liebig's Kaliapparat and
the etymology of
K and Na (1831)
Distillation and the
"Liebig"
Condenser (1771)
1828-1851 Isomerism
Radical (Dualistic) vs.
Type (Unitary, Substitution) Theory
Woehler's Urea Paper (analysis and isomerism preview) (1828)
Berzelius Coins the Term Isomeric (1830)
Woehler/Berzelius on Liebig, Isomerism, & Organic Chemistry (1830-35)
Woehler/Liebig Discovery of Benzoyl Radical (by analysis) (1832)
Dumas's Panegyric on the Radical Theory (1837)
Berzelius on Dumas Substitution Theory (1838)
Simple Alkane Etymology
Woehler's Spoof of the Type Theory (1840)
Competing Views of Organic Chemistry (~1851)
Lecture 22 - 10/27/08 (zipped) or Raw
(Radical Theory; Substitution or Type Theory)
Lecture 23 - 10/29/08 (zipped) or Raw
(Valence: Couper, Structure; Kekulé, Nomenclature, Notation, Models, Counting Isomers)
1858-1860s Constitution
"Nature and Sequence of Bonds"
Couper On a New Chemical Theory (1858)
Kirkintilloch Movie (11Mb)
Kekulé on the Superiority of his Sausage Formulae (1865)
Other Molecular Diagrams and Models (mid 1860s)
Cannizzaro, the Cautious Revolutionary, on Models (1872)
Lecture 24 - 10/31/08 (zipped) or Raw
(Counting Isomers, Cannizzaro, Koerner's Benzene Proof, Paternó's tetrahedral carbon)
Note Problems for Moday on Frame 7
1869-1880 Configuration"Arrangement of Atoms in Space"
Koerner Proves 6-fold Symmetry of Benzene (1869)
Paternó's Tetrahedral Carbons (1869)
Lecture 25 - 11/3/08 (zipped) or Raw
(Benzene Structure -van't Hoff vs. Ladenburg; Carvone/Tartaric Acid Isomerism; Pasteur, van't Hoff,)
Lieben to Paternó on Atoms in Space (1869)
van't Hoff's Tetrahedral Carbon (configuration) (1875)
Kolbe's Criticism of van't Hoff (1877)
Your Teacher, a Later-Day Kolbe?
Lecture 26 - 11/5/08 (zipped) or Raw
(van't Hoff, Tetrahedral C; Coordinate Transformation; Alkenes & Allenes; Mirrors & Alice; Chirality; Stereochemistry; Notation)
Lecture 27 - 11/7/08 (zipped) or Raw
(Configurational Notation, Fischer Projection, Counting Configurational Isomers; Systematic Nomenclature for Constitution)
Sample Nomenclature Drill (Yale only)
Lecture 28 - 11/10/08 (zipped) or Raw
(Systematic Nomenclature for Configuration - CIP; Resolution)
Stereochemical Nomenclature: Cahn-Ingold-Prelog
Chirality in the 8th Century (note Problem for Wednesday)
Lecture 29 - 11/12/08 (zipped) or Raw
( Resolution; Mechanism of Optical Rotation; Who Cares?; Chiral Drugs; Stereo Viewing; Esomeprazole)
Third Exam is coming up on Friday, Nov 14
Dr. McBride will lead a review session on Wednesday evening
9-11 PM Room 211 Mason Lab
As usual, questions will focus on material covered since the previous exam (plus topics 364 and 365 of Lecture 29),
but it is assumed that you know the material covered earlier in the course.
Remember that Lecture 19 (October 17) will also be fair game on this exam,
but the exam will NOT include most of the material from Lecture 29
(Wednesday, November 12), which will be left for the semester final exam.
Old Third Exams for Practice :
Note - we are ahead of the pace for several previous years and very slightly ahead of last year,
so different material covered in some of the earlier exams will be relevant this year.
3rd Hour Exam 2003 (pdf) ...3rd Hour Exam 2003 Answer Key (pdf)
3rd
Hour Exam 2004 (pdf) .....
3rd
Hour Exam 2004 Answer Key (pdf)
3rd Hour Exam 2006 (pdf) 3rd Hour Exam 2006 Answer Key (pdf)
3rd Hour Exam 2007 (pdf) Sorry, no answer key
(Link Repaired)
(even earlier exams available in the table below)
| | |
|
| |
|
| |
|
| |
| | |
The exam will be offered 10:15-11:15 in 111 SCL and 10:30-11:30 in 160 SCL
3rd Hour Exam 2008 (pdf) 3rd Hour Exam 2008 Answer Key (pdf)
| Exam Score Statistics | Mean | 1/3 of scores > | 2/3 of scores > |
| 3rd Exam | 76.7 | 86 | 71 |
| Sum of 3 Exams | 229.9 | 247 | 219 |
Lecture 29 - 11/12/08 (zipped) or Raw
( Resolution; Mechanism of Optical Rotation - Prof. Laurence Barron; Who Cares?; Chiral Drugs; Stereo Viewing; Esomeprazole)
Thalidomide/Ritalin
Chiral Switch
(Stereo
Viewing
this is optional, ask if you're interested)
(Omeprazole mechanism of action; Esomeprazole - Chiral Switch; Clinician Perspective - Dr. Dianne Duffey)
For timely relevance see today's editorial in the New York Times: Who Should Take a Statin
Lecture 31 - 11/19/08 (zipped) or Raw
(Esomeprazole - Litigation, Resolution & Chiral Synthesis; Conformation & Energy; Newman Projection; Rotational Barrier from Heat Capacity)
Lecture 32 - 11/21/08 (zipped) or Raw
(Source of Barrier; Stereotopicity and Toponomy; Baeyer Strain Theory; Sachse's Rhetoric;)
Topicity: Prochirality and Dehydrogenase
1885-
Conformation
"Strain"
Sachse Identifies Conformational Isomers; Baeyer objects (1890-93)
Mohr Vindicates Sachse (1918)
Drawing Perspective Views of Cyclohexane
Lecture 33 - 12/1/08
(zipped) or Raw
( Mohr's
Drawings of c-Hexane; Barton's Conformational Analysis; Ring Flips;
Rotational Barriers; Strain Energy and Molecular MechanicsAxial
vs. Equatorial Energy; Ring Relaxation; Molecular Mechanics vs.
Quantum)
Lecture 34 - 12/3/08
(zipped) or Raw
(Guest Lecture on Stereospecific Catalysis by Barry Sharpless; Molecular Mechanics of cyclobutane and cyclopentane puckering)
Lecture 35 - 12/5/08
(zipped) or Raw
(Molecular Mechanics vs.
Quantum; Generic Geometries and CSD; Energy Models; Heat of Formation;
Average Bond Energies; deltaHatomCarbon)
Lecture 36 - 12/8/08
(zipped) or Raw
(deltaHatomCarbon; "effects" in ketone-enol equilibrium; Statistics and Equilibrium)
Energy
Energies: Choice of Zero and Chupka's Heat of Atomization of Carbon
Relevant websites:
Boltzmann
Distribution
Distributions - Maxwell to Bush (powerpoint) (marginal relevance)
Entropy
& Disorder Riddle
(Statistics and Equilibrium; Potential Energy Surface; Transition State Theory; Bond Dissociation Energies; Free-Radical Halogenation; The Chemical Bond)
Multiparticle
Trajectories (Interesting, but only marginally relevant this year)
Kinetics Overview (background, with link to potential energy surface)
Eyring
Surface for H3
McBride Review Session: 8-10 pm Monday, December 15, Room 116 WLH
First Semester Final Exam
9 AM Wednesday, December 17
Davies Auditorium
(Basement of Becton Center next to SSS)
Previous
Fall Final Examinations:
Note: Some
previous
exams covered a
few more topics that we did not cover this year. You are responsible
only for what was presented in lecture this year and for assigned reading and
problems.
1999 First Semester Final
Exam
(pdf 128kB) and Answer
Key
(pdf 68kB)
2000
First Semester Final Exam
(pdf 180kB) and Answer Key (pdf 48kB)
2001 First Semester Final
Exam
(pdf 180kB) and Answer
Key
(pdf 220kB)
Supplemental information for above:
Resonance Structures ProtonatedMeOHLUMO
2002 First Semester Final
Exam
(pdf
164kB) Answer
Key
(pdf 192kB)
2003
First Semester Final Exam (pdf 380kB) (sorry no answer key)
2004
First Semester Final Exam
2005
First Semester Final Exam (sorry no answer key)
2006 First Semester Final Exam Answer Key
2007 First Semester Final Exam Answer Key2008 First Semester Final Exam Answer Key
Material for First Quarter of the Fall Semester 2008
Material for Second Quarter of the Fall Semester 2008
Material for Third Quarter of the Fall Semester 2008
Material for Fourth Quarter of the Fall Semester 2008
| |