A set of chemical transformations starting
from benzaldehyde and product analyses revealed the robust benzoyl
radical (which had been hypothesized by Lavoisier in 1789).
Wöhler and Liebig, "Studies on the Radical of Benzoic Acid"
Annalen der Pharmacie, 3,
249 (1832)
The paper begins with the following
semi-optimistic statement:
Whenever in the murky area of organic nature one
comes across a point of light that seems to offer us an entry through
which perhaps to gain the true path for investigating and
understanding it, one always has cause to count oneself fortunate,
even though one is aware of the inexhaustibility of the basic
subject.
It then proceeds to describe the
following transformations:
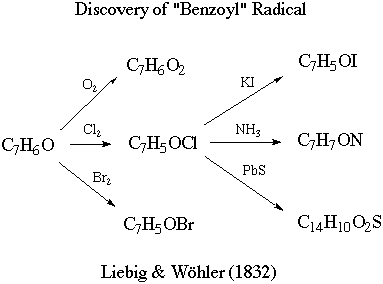
Other quotes from the paper:
Why -yl is the radical suffix. The Greek word
translitterates as ule which is how -yl sounds in German):
We call it benzoyl (the ending from υλη, substance,
matter). The resulting name for pure oil of bitter almonds would be
benzoyl hydride and for benzoic acid, benzoyl acid. Naturally we will
continue to use the old names oil of bitter almonds and benzoic acid
when it is not a question of theoretical explanations.
On pure "Benzoylhydride" [benzaldehyde] from
"bittermandelöl" [oil of bitter almonds]:
Its odor is not much different
from that of the crude oil; its taste is biting and
aromatic.
On benzoyl cyanide:
Its taste is pungent, sweet,
weaker than hydrogen cyanide.
Liebig and Wöhler lived full lives (to age 70 and 82,
respectively), but of course many 18th and 19th century chemists
suffered for their discoveries.
Click here for a lighthearted problem involving Contemporary
Relevance of a Traditional Radical
copyright 1999 J.M.McBride

