August Kekulé, Bulletin de la Société Chimique de France, 3, 98 (1865)
Footnote 2, p. 100
(2) For greater clarity I am presenting at the end of this note a table giving graphical formulae for most of the substances mentioned. The idea that these formulae are designed to express is rather well known now; so it will not be necessary to dwell upon it. I am keeping the form that I had adopted in 1859 when expressing for the first time my views on the atomic constitution of molecules. This form is nearly identical with that which M. Wurtz used in his beautiful lectures on chemical philosophy. It seems to me preferable to the modifications proposed by MM. Loschmidt and Crum-Brown.I should only remark that, for the C6A6 closed chain, I have chosen to keep the line horizontal, and that I have used arrows to represent the (terminal) affinities which are considered to undergo mutual saturation. The dots of the first two formulae indicate the unsaturated affinities.
Judge for yourself whether Kekulé's structures are preferable to those of Alexander Crum Brown, the Scotsman who, in his 1864 M.D. dissertation at Edinburgh, elaborated Couper's notation to write structures like the following for 1-propanol, 2-propanol and ethylene.
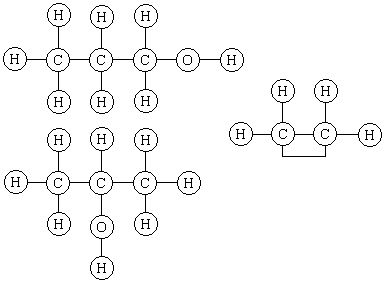
Crum Brown wrote that he "did not mean to indicate the physical, but merely the chemical position of the atoms."
If arrangement in physical space is not in question, what basis is there for preferring one notation over the other?
"In fact, in two memoirs which have appeared in Liebig's Annalen...I have put forward different views, which, in my opinion, should furnish a clearer insight into the constitution of chemical compounds."
He concludes this attack by writing
"I may be allowed to indicate that [my first paper] lays down the principle, that, besides the unknown force which is by common consent called chemical affinity, we must also, for the explanation of chemical combinations, attribute a great part to that which I have called the basicity [i.e. valence] of atoms. If Mr. Couper thinks he has discovered the cause of this difference of basicity in the existence of a special kind of affinity, I am the first to admit that I have no right to contest his priority in this."
Kekulé succeeded in his efforts to corner the glory market for discovering the tetravalence and self-binding of carbon, as well as for discovering the structure of benzene. He trained a large number of academic and industrial chemists and lived a long and honored life. Couper suffered a break-down and lived the rest of his life in obscurity at Kirkintilloch, near Glasgow, cared for by his mother. During his lifetime he never received credit for his contributions.
After Kekulé's death in 1896 his student and biographer Richard Anschutz discovered Couper's contributions and wrote in 1909,
"Without any doubt, Couper deserves the credit of having introduced into constitutional formulae the lines indicating union of atoms, and of having thus produced what are now called structural formulae."
Most chemists still refer to Couper/Crum Brown structures as "Kekulé" structures.
Go figure.