Fact,
Theory,
and
Word
Lavoisier on Combustion of Charcoal and
Phosphorus
from the "Traité" Part I, Chapter
V
This is how Lavoisier describes a combustion experiment
on carbon:
One may effect the combustion of
charcoal, like that of phosphorus, under a
glass bell jar, plate IV, figure 3, [see
below] filled with oxygen gas and inverted over
mercury : but as the heat of even red hot iron does not suffice to
ignite it, one adds to the charcoal a small piece of tinder and a bit
of phosphorus. Once can easily light the phosphorus with red hot
iron; and the flame travels successively to the tinder, then to the
charcoal.
Details on this experiment may be found in
Mémoires de l"Académie, 1781, page 448. One sees
there that 72 parts of
oxygen by weight are required to saturate
28 of
charcoal, and the the gaseous acid
produced has exactly the same weight as the sum of the weights of the
charcoal and the oxygen from which it formed.
This gaseous acid was named
fixed
air by the first chemists who discovered
it. They did not know then whether it was an air like that of the
atmosphere or another elastic fluid, vitiated and corrupted by
combustion; but since it is clear today that this aeriform substance
is an acid, that it is
formed like all other acids
by oxidation of a base, it is easy to see
that the name fixed air is not at all appropriate.
[Lavoisier and his colleagues decided to call it
carbonic acid
gas.]
Of a similar experiment with phosphorus he wrote:
Thus in this operation
45 grains of phosphorus
combined with 69.375 grains of oxygen and,
since no substance with mass could pass through the glass,
one has the right to
conclude that the weight of whatever
substance resulted from this combination and which collected in white
flakes, must
total the sum of the weighs of oxygen and
of phosphorus, that is
114.375
grains. One will soon see that these white
flakes are none other that a solid acid. [which he calls
solid phosphoric acid.]
Problems:
1) How accurate was Lavoisier's work in
these two experiments? Check his
numbers
using modern values of atomic weights.
2) How do you suppose he calculated the
weight of oxygen? Suggest a plausible source of experimental
error
(see his detailed experimental instructions below).
3) Assuming reasonable dimensions of the
apparatus (6 pint jar), how many significant figures do you think he
should have used?
4) Why do you think Lavoisier used so many
digits in "69.375 grains"?
Admire the Cleverness and Clarity of
Lavoisier's Traité Part III
"Description of Apparatus and Manual Operations in
Chemistry"
Part III , Chapter VI, Paragraph
I
On the Combustion of Phosphorus and Charcoal
To effect combustion of phorphorus or
charcoal one begins by using the pneumatochemical apparatus
over water to fill a bell jar holding at least six pints
with oxygen gas. When it is completely full and the gas
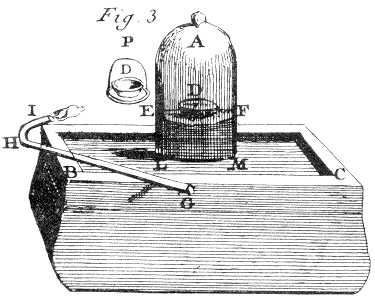
begins to bubble out, one takes the jar A to the
mercury apparatus, Plate IV, fig. 3, using a very
flat glass or china dish beneath it. Then one carefully
dries the surface of the mercury with blotting paper both
inside and outside the jar. This requires several
precautions : if one is not careful to put the paper
completely under the mercury for a certain time before
introducing it under the jar, one transfers common air that
sticks tightly to the paper.
One then takes a flat shallow dish D of
iron or porcelain containing the substance that one wants to
burn after weighing it very exactly on the balance ; one
then covers this dish with a slightly larger one
P,which serves as a diving bell, one transfers the
assembly through the mercury ; after which one brings back
P, which served only as a cover. One can avoid the
challenge and difficulty of passing materials through the
mercury by lifting one side of the bell jar for a tiny
instant and thus introducing the dish with the combustible
material through the gap. Using this second approach a
little common air mixes with the oxygen gas ; but this
mixing, which is slight, damages neither the success nor the
precision of the experiment.
|
|
When dish D has been introduced under the bell jar, one sucks
out part of the oxygen gas that it contains to raise the mercury to
EF. Without this precaution, once the combustible substance is
lit, the heat expands the air ; part of it would escape the bell jar
and one could make no exact calculation on the quantities. To suck
out the air one use the siphon GHI, which one sticks under the
bell jar ; and to keep it from filling with mercury one twists a
small piece of paper around its end I.
There is a way of raising a column of mercury
several inches above its level by sucking
[Note: this approach is not sanctioned by current standards of
safe laboratory practice] : if one sucks
air with the lungs, one can achieve only a very mediocre elevation,
for example an inch or an inch and a half at most ; and then only
with extraordinary effort ; while by the action of the muscles of the
mouth one can raise the mercury to six or seven inches without
tiring, or at least without risk of trouble. A more convenient method
still is to use a little pump attached to the siphon GHI ; then one
can raise the mercury to whatever appropriate height as long as it
does not exceed 28 inches.
|
If the combustible substance is very
inflammable, like phosphorus, one lights it with a curved
iron rod, plate IV, fig. 16, heated red in the fire,
and poked quickly under the bell jar : as soon as it touches
the phosphorus, it lights. For less combustible substances,
such as iron, several other metals, charcoal, etc. one uses
a bit of tinder on which one places a speck of phosphorus :
and lights the last in the same way with the curved rod ;
flame travelling to the tinder and then to the combustible
substance.
|

|
In the first instant of combustion the air expands and the mercury
descends; but when no elastic fluid
[gas] is formed, as in combustion of iron
or phosphorus, absorption soon becomes observable and the mercury
climbs high in the jar. Thus one must be careful not to burn too much
of the combustible substance in a given quantity of air; otherwise
near the end of combustion the dish approaches the dome of the jar
too closely and the great heat could make it crack.
In Chapter II, paragraphs V and VI, I presented
ways of measuring the volume of gases and necessary corrections of
the volume relative to the height of the barometer and the
temperature ; I will add nothing in this regard. The example cited on
p. 381 was taken from the combustion of phosphorus.
[Measuring the amount of oxygen consumed is easier for
phosphorous, which gives a solid, than for carbon, which gives a gas
of the same volume as that of the oxygen consumed. Lavoisier could
have absorbed the carbon dioxide with solid potassium hydroxide to
determine the reduction in volume of oxygen.]
...this method is not without danger for
substances which are susceptible to vaporizing with modest heat, such
as ether, wine spirits, and essential oils. These volatile substances
dissolve in a rather great quantity in oxygen gas ; when one lights
them, there is a sudden detonation which blows the bell jar to a
great height and shatters it. I have observed two of these
detonations, of which members of the Academy have, like myself, seen
themselves to be the victims. [obviously this must also
have scattered mercury far and wide.]
copyright 2000
J.M.McBride