If one could change the conformation freely so as to
consider only time-averaged environments
(i.e. consider only configurational stereotopicity),
what would the relationships between the various pairs of hydrogens be?
Topicity and Yeast Alcohol Dehydrogenase
|
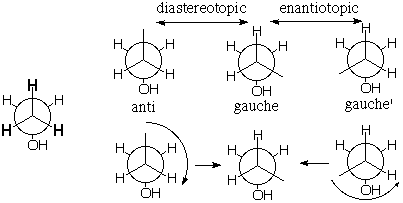
Consider the topochemical relationship among the methyl (CH3) protons of ethanol. The gauche proton whose site (i.e. environment) is shown in the middle structure is diastereotopic with the anti proton (on the left) and enantiotopic with the gauche' proton (on the right). That is, the sites are diastereomeric and enantiomeric, respectively. However, it is a little silly to make very much out of these differences because, as shown below, 120° rotation of the methyl group interconverts these sites, and it will occur VERY rapidly [barrier <4 kcal/mole means that the rate will be greater than 1013/sec x 10-3/4 x 4 = 1010 per second]. Pairs of methyl protons are only conformationally heterotopic (diastereotopic or enantiotopic). [Note: Of course we should ignore the absence of symmetry created by the way the OH group is drawn. Its H would actually be pointing back into the page rather than to the right and it would wag right and left equally often.] |
|
|
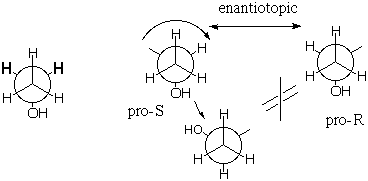
Now consider the methylene (CH2) protons. Like the gauche and gauche' protons above they are enantiotopic with one another, BUT their sites are NOT interconverted by rotation of the back carbon about the C-C bond. The methylene proton sites are mirror images and the protons are configurationally enantiotopic. Note that in naming the methylene protons, we promote the site whose proton is to be named to higher priority than its enantiotopic mate. |
|
Why do we care?
The enantiotopic pro-R and pro-S methylene protons, being mirror images, should share all properties except for interaction with a single enantiomer of a chiral substance. If such a chiral substance interacts with the molecule, the sites (which now include the new substance) cannot be mirror images of one another, even when time averaged, because the new reagent located next to the pro-R hydrogen is NOT the mirror image of the new reagent located next to the pro-S hydrogen (because the new reagent cannot be its own mirror image if it is chiral). With the new chiral molecule present, the methylene hydrogens become diastereotopic rather than enantiotopic, and they will behave differently, e.g. one could react more readily than the other. Note in the figure below that the mirror image (equal energy) of a right hand reacting with the pro-R hydrogen, is not the right hand reacting with the pro-S hydrogen, but the pro-S hydrogen reacting with the left hand. If the chiral substance is resolved and contains only right hands, the enantiotopic hydrogens should behave differently.
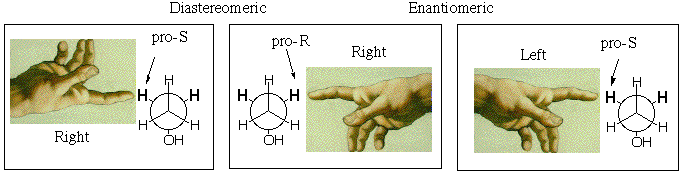
Natural enzymes are chiral and come as a single enantiomer (like right hands), so they should be able to discriminate between the enantiotopic hydrogens. For example Liver Alcohol Dehydrogenase (LAD) removes one methylene hydrogen and one OH hydrogen from ethanol during its metabolism (it doesn't really give H2, but more of that next semester):
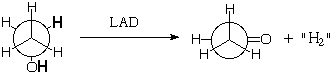
Might LAD specifically remove one of the methylene hydrogens preferentially (as shown)? Probably, but how could you prove it?
You could prove that LAD removes only the pro-R hydrogen by starting with ethanol whose pro-S hydrogen is replaced by deuterium and showing that the aldehyde product contains only deuterium, which would have been lost from the aldehyde to the extent that the pro-S hydrogen was abstracted. (You could use nmr spectroscopy to see how much deuterium is in the aldehyde.)
But where in the world would you obtain resolved mono-deutero ethanol?
If the enzyme catalyst does indeed operate specifically, you could get it by running the dehydrogenation backward using the Law of Mass Action! You could start from aldehyde containing deuterium in the presence of a big excess of what we are calling "H2" to generate the "deuterium-labeled" ethanol, then react the labeled ethanol with LAD in the absence of "H2" to make the reaction run in the normal direction. If the enzyme is not specific, it will introduce H randomly in the pro-R and pro-S methylene positions, and them remove H and D at random, so that only about half the ultimate aldehyde product will still contain the original deuterium. If the enzyme is specific, it will put on H in the pro-R position, and then remove it again, leaving all of the original deuterium with the final aldehyde.
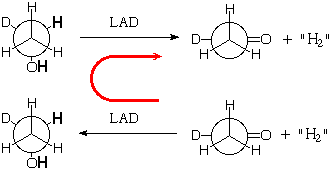
This works!
If you run the second reaction in the presence of a lot of "H2" then the first reaction in the absence of "H2" you in fact find that all of the original deuterium is retained by the aldehyde. So the enzyme is perfectly specific in selecting between the enantiotopic hydrogens.
However the result would have been exactly the same if the enzyme had specifically donated H to, and then removed it from, the pro-S position. It was more work to show which position it works with.
One neat trick is to use one enzyme to add the H2 and another one to remove H2 (or HD). This approach is able to divide enzymes into two classes, those that work on the pro-R hydrogen and those that work on the pro-S. There are a number of examples of each.
One could clearly consider them Constitutional Homomers ("Finger bone connected to the hand bone; hand bone connected to the wrist bone," etc.).
How about from the point of view of Configuration and Conformation?
(Answer)
|
|
copyright 2001 J.M.McBride