Systematic Stereochemical Nomenclature &
Vladimir Prelog
To the Student: Sections 1 and 2 are the most
substantive portion of this page for a course in organic
chemistry. The other sections may help to make this topic
memorable and should contribute insight on the character and
personalities of several leading organic chemists of the 20th
Century
1) Stereochemistry
~1950
2) Cahn, Ingold,
Prelog
3) Buergenstock
Declaration
4) Remembering
Prelog
5) Prelog Stories
- - - Robinson
& Woodward
- - - Ruzicka as
Administrator
- - - The Montenegran
Peasant
|
Just at the midpoint of
the 20th Century there were three important developments
in stereochemistry.
The first was signaled by Derek
H.R. Barton's
paper "The Conformation of the Steroid Nucleus"
(1950), which brought serious consideration of
conformation
of organic molecules to centerstage and ultimately led to
Barton's Nobel Prize.
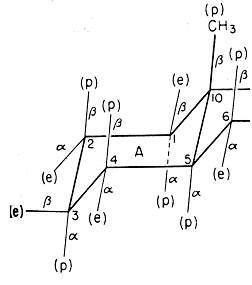
The figure at the right, from
Barton's 1950 paper, shows the chair conformation of the
"A" ring of a steroid molecule. A change since 1950 is
that we now call one set of substituents "axial" instead
of "polar" (p), though we still use Barton's "equatorial"
for the other set. A more significant change is our
understanding of the correct directions for the bonds.
What is wrong with Barton's figure?
(click
for answer)
|
|
(Bijvoet's Tartaric Acid)
|
The second important development
was a 1951 paper (announced in 1949) by Johannes
Martin Bijvoet (pronounced "buy foot" 1892-1980) a Dutch
crystallographer. In "Determination of the Absolute
Configuration of Optically Active Compounds by Means of
X-Rays" Bijvoet reported that using the sodium/rubidium
salt of (wouldn't you know) tartaric acid and a special
property of the heavy Rb atom (called "anomalous
dispersion") he was able to do
what no one had been able to do before - prove which
enantiomer of a chiral compound was which. He showed that,
given a 50:50 chance, Emil Fischer had guessed right when he
drew the Fischer projection of tartaric acid 58 years
earlier. From this time forward it would be possible to draw
and name compounds according to their actual, or
"absolute",
configuration,
rather than their configuration relative to an arbitrary
standard (like Fischer's
D-glyceraldehyde).
|
The third important development at
mid-century involved
nomenclature.
As Bijvoet wrote in 1951,
The question of nomenclature is beyond
the scope of our investigation... The problem of nomenclature now
concerns given configurations, and requires a notation which
denotes these configurations in an unambiguous and if possible
self-explanatory way.
Although conventions existed for
drawing unambiguous diagrams of chiral molecules, there was no
simple, systematic, unambiguous nomenclature for them. This need was
addressed in a 1950 paper by two Englishmen, R. S. Cahn
(1899-1981), editor of the Journal of the Chemical Society (of
London), and Christopher Ingold (1893-1970) of
University College London, who was a leader in discovering how
reactions occur (in fact he studied, and named, the
SN1
and
SN2
substitution processes, which we will soon discuss). Their paper,
"Specification of Configuration about Quadricovalent Asymmetric
Atoms", proposed a scheme based on a sequence rule
of atom priorities. As Lavoisier would have predicted, the
availability of an appropriate nomenclature has made working,
thinking, and communicating in this field much more efficient and
productive.
|
In 1955 the scheme of Cahn and Ingold was
expanded and generalized in collaboration with
Vladimir Prelog of the Swiss Federal Institute
of Technology (ETH), Zurich. Prelog had impressive
credentials from accomplishments in synthesizing antibiotics
and other natural products and would receive the Nobel Prize
in 1975 "for his research into the stereochemistry of
organic molecules and reactions." His logical insights
improved the R/S nomenclature scheme, which is now
known as
CIP
(Cahn, Ingold, Prelog). Just as importantly Prelog's sense
of humor and personal magnetism facilitated quick acceptance
of the scheme.
Every spring since 1966 a conference on
stereochemistry has taken place at Bürgenstock, a Swiss
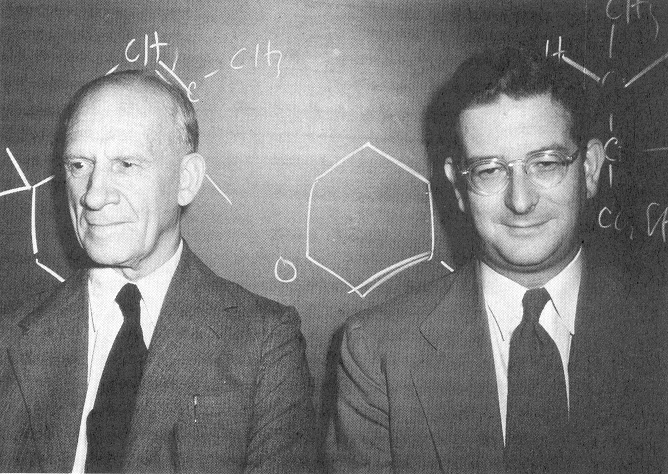
resort overlooking the Lake of Lucerne. The photograph at
the right shows Cahn, Ingold, and Prelog (l to
r) at the first conference.
|

(thanks to Jeffrey Seeman for this
photo)
|
|
The "Buergenstock Declaration,"
generated at the 1966 session of the Bürgenstock
conference, was signed by C, I, and P. (reproduced
by permission of the scribe and coauthor, J. D.
Dunitz)
|

|

Problem: interpret
Prelog's signature on this document
If you give up on deciphering his (Cyrillic?) signature,
click here.
|
|
Full of honors,
Vladimir Prelog, whom his myriad friends called Vlado, died
on January 3, 1998, at the age of 91.
|
Prelog's
capsule Nobel autobiography and
his book "My 132 Semesters of Chemistry Studies"
(J. Seeman, ed., American Chemical Society, 1991,
from which two stories and the figures were
taken) reveal a person of keen intelligence,
irresistible charm, and deep wisdom. The title of the book
refers to his ruse for staying on at the ETH after reaching
the compulsory retirement age. He enrolled as a student. In
these accounts:
• You meet a citizen of the
world who, as an 8-year old in Sarajevo, was waiting to
scatter his little basket of flowers in front of the
carriage carrying Archduke Franz Ferdinand and his
duchess, when a few hundred meters away they were
assassinated, triggering World War I.
• You learn of
his admiration for Robert
Robinson, Ingold,
and Leopold
Ruzicka (pronounced "Roo
zheets ka"). All three of them would probably have been
Nobel laureates had not Robinson's influence in Sweden
discouraged an award to Ingold, his chemical foe and
fellow countryman.
(Robinson, of Oxford, was a leader in the
synthesis of natural products. He introduced the use of
curved arrows to denote electron pair shifts. He
also invented the descriptive term "mesomerism",
which unfortunately lost out to the confusing term
"resonance" leading to the consternation of generations
of chemistry students. He considered that Ingold had
appropriated the curved arrow without proper
acknowledgement of Robinson's priority. Next semester we
will discuss the clever Robinson Annulation
reaction.)
• You learn how Ruzicka, another
Croat and a leader in the structural determination and
synthesis of hormones, brought Prelog to safe haven in
Switzerland during World War II, and how Prelog succeeded
Ruzicka as director of organic chemistry at the ETH, one
of the world's most respected laboratories.
|
|
To appreciate him as a person, you
have to hear
Prelog's
stories. Four of them appear
below. The first two are from his autobiography and concern
Sir Robert
Robinson and
R.
B.
Woodward.
|
|
|
Robinson and Woodward after a
1951 MIT seminar by Robinson
on the Robinson annelation
in Steroid Synthesis
(photo J.D. Roberts, by permission)
|
|
Robinson on
Systematic Nomenclature
As I already mentioned, Robinson disliked
the CIP
system for the specification of
molecular chirality. When we once met at Zurich airport on
the way to Israel to celebrate the 25th anniversary of the
Weizmann Institute, the first words we exchanged were the
following:
Robinson: "Hello
Katchalsky. What are you doing here in Zurich?"
I: "Excuse me, Sir Robert,
I am only Prelog, and I live here."
Robinson: "You know,
Prelog, your and Ingold's configurational notation is all
wrong."
I: "Sir Robert, it can't be
wrong. It is just
a convention. You either accept it
or not."
Robinson: "Well then, if it
is not wrong, it is absolutely unnecessary."
|
|
Woodward on Robinson on
Prelog
Woodward and Sir Robert Robinson held
each other in high esteem, but they did not always get along
well. One day, probably in the late 1950s, when Woodward
visited us in Zurich he said to me,
"You know, Sir Robert is a bad
old man."
I: "How can you say
something like that about the greatest living organic
chemist?"
Woodward: "Yesterday, I
spent the whole day with him in Oxford. He doesn't
communicate much with me any more about chemistry, but he
does talk about individuals. He didn't find a single good
word for any chemist in the whole world."
I: "Perhaps he was in a bad
mood; that doesn't mean that he is evil."
Woodward, irritated by my contradicting,
replied, "Indeed, I didn't tell the
truth. He said something nice about you."
I suspecting something wrong, asked,
"What did he say?"
And Woodward triumphantly replied,
"Prelog is a lousy chemist, but he is a
rather nice guy."
How true!
[the "How true" is Prelog's and
part of the story. Prelog painstakingly reworked these
two stories to make sure they were just right for his
published autobiography. He decided on a bowdlerized
punch line "Prelog is not a good chemist, but he is a
nice person." On the advice of his friends I have
retained a version quoted by Heilbronner in A
Philatelic Ramble through
Chemistry
(Wiley-VCH
1998).]
|
A resident of many countries, including Croatia,
Czechoslovakia, and Switzerland, and a frequent visitor to Britain
and the U.S., Prelog was skilled in his use of foreign languages, but
self-deprecating about it. He loved telling stories, and how he told
them was part of his charm and humor.
|
Prelog with his mentor
Ruzicka
in Zurich, 1953
(photo J. D. Roberts, used by
permission)
|

|
Click to
hear Prelog tell
Leopold
Ruzicka as an
Administrator
(recorded in 1992, and transcribed below):
(When you click above in a properly configured
browser, a small sound control window
should appear. It may be hidden behind this window, and
you may also need to
adjust the volume elsewhere. There are pedantic
notes for the words in italics.)
|
I think chemical research costs now about 10 to
100 times as much as it did in '45.
You know, we bought Perkin-Elmer.
Thompson was here and so on, so Ruzicka said, "We must have
Perkin-Elmer" - infrared.
Perkin-Elmer was 60,000 Francs. It was unheard.
Nobody was able to give so much money. So the school gave 20,000
Francs, and then industry gave 20,000 Francs, and still 20,000
Francs were lacking.
But there is a foundation here, of our school,
which from time to time can spend some money. So at the sitting of
this foundation they decided to give Ruzicka the last 20,000
Francs.
And he had many people - the people didn't like
him. The chair was Professor Frey-Wyssling, and he was
president of the school and so forth, or expert and so. So this
Frey-Wyssling told Ruzicka, "Ruzicka, hier bekennen Sie. (You have
to confess.) You ordered this machine before we decided to give
you this 20,000 Francs."
And Ruzicka said, "You don't know me at all.
The machine is already half a year working."
And then the President took Ruzicka and said,
"Professor Ruzicka, you shouldn't say such things before the
public. Your financial activity is not a solid one."
And he said, "Oh, I did it for 60 years,
and I was always very glücklich (happy)"
And the President said, "Ruzicka, you don't
still speak well German. You were not happy; you were lucky." (He
said, "You were not glücklich, you were
glückhaft.")
Here is a final Prelog story about his attitude
toward the Nazis, who occupied Zagreb when he was a lecturer there in
1941, forcing him and his wife to flee to
Switzerland:
Montenegro is the poorest part of the
Balkans. Early this century there was a Montenegran peasant who
had nothing but a sickly goat. One day a wolf came out of the
forest and ate the goat.
The peasant had no choice but to emigrate to
Italy, where by menial jobs he earned passage to Canada. Finally
he arrived in the United States, where he found a job stocking
shelves in a grocery store. When by hard work and frugal living he
had saved a little money, he bought part interest in the store.
Eventually he was able to buy the grocery store outright, and
finally to acquire a whole chain of grocery stores.
Although he had become very successful, he
never forgot his roots. Faithfully each year on his name day he
went to the Orthodox church to light two candles - one for his
patron saint, and one for the wolf.
Pedantic
Notes
Perkin-Elmer:
An instrument company founded in 1937 in Norwalk, Connecticut, as an
American source of precision optics. Perkin-Elmer entered the
emerging field of analytical instrumentation in the 1940s. For many
organic chemists Perkin-Elmer was synonymous with recording IR
spectrometer.
Thompson: Harold W. (Tommy) Thompson
(1908-1983), of Oxford, pioneered the application of IR spectroscopy
for organic chemical analysis. In his 1944 Tilden Lecture "The
Scope and Limitations of Infra-red Measurements in Chemistry", he
modestly wrote,
It seems probably that
infra-red analysis will soon rank highly for routine and
research work in organic chemistry, where its usefulness may at
least equal that of ultra-violet spectroscopy.
[Thompson was using IR to analyze German
high-performance aviation fuel. This fine lecture was published
in 1944 on pages 183-192 of the thin 723-page wartime volume of
Journal of the Chemical Society (London). In 1938 this
journal had 2200 pages; in 1948, 2375. ]
After the war Thompson gave a series of lectures
on IR in Zurich. He was obviously a good ambassador and salesman for
this technique. (For an obituary notice, pointing out that Sir
Harold also had a "major impact on association football" see Rex
Richards, Chemistry in Britain, 1985, 21, 941)
Frey-Wyssling: Professor Albert
Frey-Wyssling (1900-1990) a distinguished botanist with an interest
in structure was a foreign member of the U.S. National Academy of
Science. He made some of the first x-ray diffraction studies of
cellulose.
The chair was: This is filler. No
one has yet made out the few words in here.
60 years: This was Ruzicka's age at
the time, i.e. he has been doing this for his whole life.
Prelog seems to toy with the alternative of 50 years (as a very young
child Ruzicka may have been better behaved).
I am indebeted to Jack Dunitz, Albert
Eschenmoser, and Claude Wintner for much of this information and for
their help in transcribing "Ruzicka as Administrator".
Return to
Chem 125 Home Page
copyright 1999/2002 J. M.
McBride
