Recognizing
Functional
Groups
Molecules are reactive when they have groupings of atoms that
give
rise to unusually high-energy HOMOs or unusually low-energy LUMOs.
Understanding the causes of such unusual orbitals allows one to
recognize "functional" groups and to predict which functional groups
should react with which other functional groups. SOMOs react with
SOMOs, such that a molecule with a SOMO can react with an identical
molecule. However, high HOMOs react not with other high HOMOs, but
with low LUMOs, and vice versa.
|
Some of the most valuable generalizations in chemistry
have involved the concepts of acids and bases and their mutual
reactivity.
In 1887 the Swedish chemist Svante Arrhenius
proposed the revolutionary idea that salts could ionize in solution. He
further proposed that acids ionized to give H+,
while bases gave OH-.
In 1923 the Dane Johannes Brønsted
and the Englishman Thomas Lowry generalized the idea of base beyond OH-
to include any proton acceptor, for example NH3.
G. N. Lewis expanded these categories further by
recognizing that all bases were electron-pair donors and that H+ was
not the only electron-pair acceptor. Other species can also accept a
share in a pair of electrons to complete their octet, for example BH3.
Now we realize that molecules are electron-pair donors
when they have unusually high-energy HOMOs and are electron-pair
acceptors when they have unusually low-energy LUMOs.
|
Theory
|
Acid
|
Base
|
|
Arrhenius
|
H+ Source
|
OH- Source
|
|
Brønsted / Lowry
|
H+ Donor
|
H+ Acceptor
|
|
Lewis
|
e-Pair Acceptor
"Electrophile"
|
e-Pair Donor
"Nucleophile"
|
|
HOMO/LUMO
|
low LUMO
|
high HOMO
|
So to understand reactivity we need to be able to recognize
unusually high HOMOs and unusually low LUMOs. Every molecule has a
HOMO and a LUMO, the key word is "unusual."
|
Something is unusual only in comparison to what is
usual. We have to decide what "usual" is.
Our benchmarks for usual HOMO and
LUMO energies are those of the garden-variety unreactive molecules of
organic chemistry, the alkanes. These are the occupied σ
orbital and the unoccupied σ* orbital of single bonds like H-C
and C-C, the kind of bonds that occur in alkanes.
|
|
This recalls an old vaudeville routine, which we
might update as follows:
Two friends meet at the health club and
begin chatting.
Marilyn asks Pauline, "How's your husband?"
Pauline replies, "Compared to what?"
"Compared to what?" is the
second most important question we address this year. The most important
is "How do you know?"
|
|
[One might worry that
individual H-C and C-C molecular orbitals within the same alkane
molecule might mix with one another to give composite higher- energy
HOMOs and lower-energy LUMOs. But the overlap between adjacent H-C and
C-C MOs is very small, typically less than 10% as large as the overlap
that generated these sigma bonds and antibonds in the first place. Thus
even it they do mix, there is no substantial influence on the orbital
energies. There are appreciable effects for the π (and π*) MOs
of double bonds. We'll worry about this later on when we talk about
"conjugation".]
In C-H and C-C there is good overlap and good energy match of
the
atomic orbitals, which results in substantial splitting between the
σ HOMO and σ*
LUMO in this "normal" case.
|
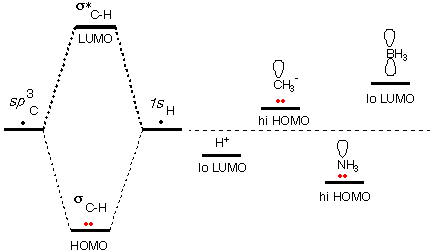
There are a number of cases where species are acids (or
bases) because the LUMO (or HOMO) is an atomic orbital that has not
been mixed with another atomic orbital to form a bond and still has its
unshifted atomic energy. [The energy levels in the diagram to the right
are schematic. Don't try to interpret them quantitatively.]
Two examples of surviving atomic orbitals are the 1s
LUMO of H+ and the sp~3
HOMO of CH3-.
[Note that the + charge makes the H+ LUMO even
lower in energy (a better home for electrons), and the negative change
makes the CH3- HOMO even
higher in energy. Here we are taking electron repulsion into account in
a way.]
Because of the higher nuclear charge, an atomic orbital
on N is lower in energy than a corresponding orbital on C. But the
unshared pair orbital on NH3
is still an unusually high HOMO, because our standard is not a C atomic
orbital, but the σC-H
bonding orbital. The N pair has not been stabilized by overlap with
another AO.
Because of the lower nuclear charge, an atomic orbital
on B is higher in energy than a corresponding orbital on C. But the
vacant atomic orbital on BH3
is still an unusually low LUMO, because our standard is not a C atomic
orbital, but the σC-H
antibonding orbital. The p-orbital on B has not
been destabilized by overlap with another AO.
|
|
Unshared pairs on oxygen make H2O
basic for the
same reason that NH3 is. But H2O
is a much
weaker base (lower energy HOMO), because oxygen's greater nuclear
charge make its AOs lower in energy than those on nitrogen. On the
other hand OH- is a stronger
base than
NH3, because the negative charge raises the
energy of its
unshared pairs. Of course OH- is not as strong a
base as
CH3-, because of the
difference in nuclear
charge.
So the first
source of
unusual orbitals is the survival of
valence-shell atomic
orbitals as vacant orbitals or unshared pairs.
The energy
of these orbitals is influenced by nuclear and molecular charge.
PROBLEM: How would you explain that Li+
is not
very acidic and F- is not very basic?
|
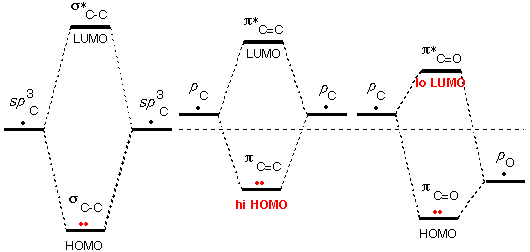
A second
source of unusual HOMO/LUMO energies is
molecular orbitals that result from poor
overlap between atomic orbitals (the orbitals in
the previous examples had no overlap at all).
As
we saw previously, the side-to-side overlap of
atomic p orbitals is only about half as
effective as the end-to-end overlap of atomic hybrid orbitals. Thus the
energies of the unusual π
and π* MOs are not
"split" by as much as those of "usual" σ
and σ* MOs.
In the case of the C=C double bond, p
AOs are used to form these MOs. This high-energy start makes the π MO particularly unusual, so
that the C=C group is basic and normally attacked by acids.
In the case of the C=O double bond, p
AO of oxygen is much lower in energy than the AOs used to form the C-C
bond, this low-energy start makes the π* MO particularly unusual, so that the C=O group is
acidic and normally attacked by nucleophiles (Bases are called
"nucleophiles" when they attack atoms other than hydrogen).
There can be other reasons for poor overlap -
for example when the atomic hybrids of carbon do not point directly
toward one another to achieve maximal overlap. This gives
three-membered-ring hydrocarbons high HOMOs and makes them reactive
with acids (remember the x-ray evidence for bent bonds?).
|
|
|
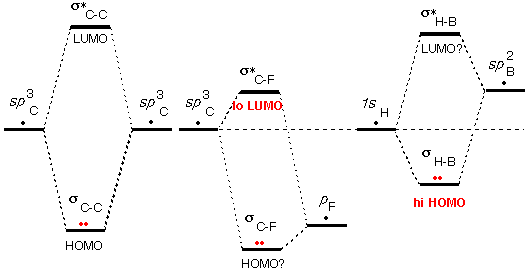
The third
source of unusual HOMO/LUMO energies (and the
last) is that one or more
of the AOs used in making the MOs is higher or lower in energy
than the valence orbitals of carbon and hydrogen.
In fact this factor was already invoked above
in deciding that the HOMO of C=C and the LUMO of C=O were particularly
unusual.
As shown schematically on the right, the low energy of
the F valence orbital (high nuclear charge) makes σ*C-F
an unusually low LUMO. Carbon-halogen bonds are subject to attack by
bases (high HOMOs).
By similar reasoning the high energy of the Li valence
orbital (low nuclear charge) makes σC-Li
an unusually high HOMO, susceptible to attack by acids (low LUMOs).
Most such "organometallic" compounds are reactive with acids.
Note that since boron has a lower nuclear charge than
carbon, the sp2B
orbital is higher than the 1s orbital of hydrogen.
This makes σB-H
an unusually high HOMO and makes BH3 a base.
Above we saw that because of the vacant p orbital
on B, BH3 is also an acid. This means that two BH3
molecules should react with one another, and indeed they do react to
generate B2H6.
|
|
You should be able to use these three considerations
(presence of valence-shell AOs, poor overlap, unusual AO components) to
identify what kind of reactivity (as acids or bases) the functional
groups in Table 3.1 should exhibit.
copyright 2001 J. M. McBride


