
|
|
|
|
1/3 of the scores were greater than 82 2/3 of the scores were greater than 71 |
1/3 of the scores were greater than 161 2/3 of the scores were greater than 147 |
If you haven't done as well as you should so far, don't lose heart. If you've been cruising, don't become complacent. The first two exams count for only 1/3 of the semester grade. The remaining hour exam counts for 1/6 and the final exam for 1/2. It makes much more difference how you do from here on out that what scores you've gotten on the first two exams.
What is important is that you master the material thus far, because the rest of the course will build on it. Students who scored 50 or less on this exam or 125 or less on the first two exams should contact Justin about special help sessions.
If we had to assign letter grades at this point (which we don't) the line between A and B would be about 153, and the line between B and C would be about 110.
1. (5 minutes) What is an orbital?

2. (4 minutes) Overlap Integrals (net overlap)
a. What orbital hybridization of carbon gives
the best overlap at the C-C single bond distance?
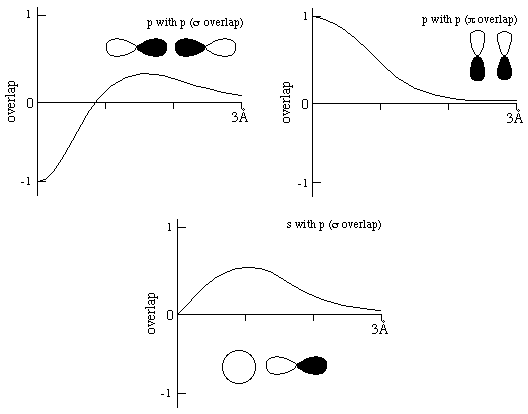
b. Put two numbers for scale on the vertical axis of each of the following graphs, and sketch how the overlap integral for the indicated orbitals varies with the distance between two carbon atoms:
3. (4 minutes) Explain in mathematical terms why overlap is important in interpreting the simplest MO for H2.

4. (7 minutes) Draw orbital shape (not energy) and curved arrow diagrams (two separate diagrams) to show the reaction of NH3 with H2C=O. Use a few words to explain the position from which NH3 attacks. (Prof. Dunitz sends his regards from Zurich, I am writing this from his office.)

5. (5 minutes) Give two reasons why one is justified in neglecting "core" (1s) orbitals in discussing the bonding of non-hydrogen atoms.

6. (6 minutes) One might consider the following possibilities for resonance stabilization of ethyl cation and of ethyl chloride. Beneath each pair of structures draw the shape of the orbitals of the structure on the left that mix to make the structure on the right a plausible resonance structure. Name the orbitals that mix, and use a few words to explain the change of atom-atom attraction upon orbital mixing.
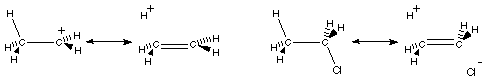

7. (6 minutes) For each of the two compounds in Question 6 draw an energy diagram showing the mixing of localized orbitals of the structrure on the left that gives rise to the "no bond" resonance structure on the right. Label each energy level. Draw the diagrams side-by-side with the same energy scale using the horizontal dotted line as the valence orbital energy of an isolated C atom (neglect difference between 2s and 2p). Explain differences between the two diagrams to show which compound has more important resonance stabilization.
|
|
|

 | |
8. (13 minutes) Simple alkanes do not normally react with acids, but the C-C bonds of cyclopropane can react with HBr to give n-propyl bromide in a two-stage reaction.
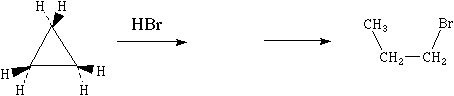
(A) Explain why cyclopropane contains an unusually high HOMO. [Hint: draw a picture that shows the angles between the sp3 hybrid AOs of carbon that overlap to form the C-C bonds in cyclopropane]
(B) Name the LUMO of HBr with which cyclopropane's HOMO mixes in the first step of the reaction. What makes it a low LUMO?
(C) Use curved arrows to show both steps involved in the conversion of cyclopropane to n-propyl bromide.
Answers
1. (5 minutes) What is an orbital?
Why is it fundamentally incorrect to express a molecular wave function in terms of orbitals? (Mention probabilities in your answer)
A number of students got off the track by talking about the square of a single orbital - expressed as an LCAO - which gives the overlap products, rather than about the square of a product of orbitals for different electrons. Although a particular simple formulation of the AO may be erroneous, there is no fundamental problem with the concept of an AO for a one-electron problem; it is only with two electrons that the idea as a whole breaks down.
2. (4 minutes) Overlap Integrals (net overlap)
sp - sp (as shown on handout, 2 points)
b. Put two numbers for scale on the vertical axis of each of the following graphs, and sketch how the overlap integral for the indicated orbitals varies with the distance between two carbon atoms:
Crucial features of these answers are:
That the curves start at -1, 1, and zero, respectively
That all curves begin to hunker down toward zero by 3 Å (but don't approach it abruptly at a point)
That p-p sigma overlap changes sign before the C-C bond region of 1.2-1.6Å.
Less important for present purposes are:
The detailed shape of the curves (the following are only approximate)
That the overlap never gets very close to 1 in the bonding region.
The relative sizes of the overlaps
3. (4 minutes) Explain in mathematical terms why overlap is important in interpreting the simplest MO for H2.
which when squared gives the electron probability density
The first two terms in the sum are just the undistorted atomic electron densities. It is the product AB term that generates a bond, shifting electron density to regions between the atoms where both A and B have significant values. If there is no overlap, there is no shift of electron density to the bonding region. If the overlap integral is zero by symmetry (A and B are orthogonal) then the density would shift toward one bonding region exactly to the same extent that it shifts away from an equivalent region, giving no net stabilization
4. (7 minutes) Draw orbital shape (not energy) and curved arrow diagrams (two separate diagrams) to show the reaction of NH3 with H2C=O. Use a few words to explain the position from which NH3 attacks.
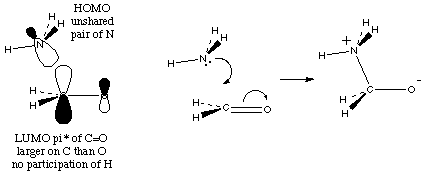
To generate maximum overlap NH3 attacks the LUMO on C slightly away from the O with which its overlap would be negative.
(Note: N can't approach opposite the O without running into occupied orbitals involving hydrogen; the 1s orbitals of H do not participate in the LUMO, since they are orthogonal to this pi-type orbital; to show reaction, you should draw the two reacting orbitals in the orientation where they are beginning to overlap)
5. (5 minutes) Give two reasons why one is justified in neglecting "core" (1s) orbitals in discussing the bonding of non-hydrogen atoms.
The core orbitals are held so close to the nuclei that they have very little overlap with valence orbitals of other atoms.
The core orbitals are so low in energy that they have gross mismatch with valence orbitals of other atoms and therefore negligible mixing. In principal they could mix extensively with the core orbitals of the same energy on other atoms, despite poor overlap, but since both orbitals are fully occupied by elecron pairs, there is negligible overall change in energy from the mixing (even the small net repulsion is negligible, because ovelap is so small).
6. (6 minutes) One might consider the following possibilities for resonance stabilization of ethyl cation and of ethyl chloride. Beneath each pair of structures draw the shape of the orbitals of the structure on the left that mix to make the structure on the right a plausible resonance structure. Name the orbitals that mix, and use a few words to explain the change of bonding upon orbital mixing.
|
| |
|
Mixing the filled sCH with the vacant pC+ weakens the CH bond by removing some of its bonding electrons and strengthens the C-C bond by building up electron density in the p overlap region between the carbons. |
Mixing the filled sCH with the vacant s*CCl weakens the CH bond by removing some of its bonding electrons, weakens C-Cl by introducing antibonding electrons, and strengthens C-C by building up electron density in C-C p overlap region. |
7. (6 minutes) For each of the two compounds in Question 8 draw an energy diagram showing the mixing of orbitals of the structure on the left that gives rise to the "no bond" resonance structure on the right. Label each energy level. Draw the diagrams side-by-side with the same energy scale using the horizontal dotted line as the valence orbital energy of an isolated C atom (neglect difference between 2s and 2p). Explain the differences between the two diagrams to show which compound has more important resonance stabilization.
|
|
|
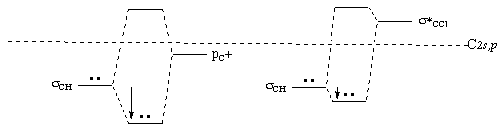 | |
Resonance is more important for the ethyl cation, because it shows more stabilization of the electron pair upon intramolecular "HOMO/LUMO" mixing. This is because there is better energy match between the "HOMO" sCH orbital (which has been stabilized from the atomic C energy (horizontal line) because of strong bonding) and the "LUMO" pC+ (which has been lowered from the atomic C energy because of the + charge) than between the "HOMO" sCH orbital and the "LUMO" s*CCl orbital (which has been raised from the atomic C energy by mixing with the atomic orbital of chlorine to form the C-Cl bond). [Note that it is not just the energy shift that makes resonance more important for the ethyl cation, there is also a greater shift of electron density on forming the mixed orbital.]
8. (13 minutes) Simple alkanes do not normally react with acids, but the C-C bonds of cyclopropane can react with HBr to give n-propyl bromide in a two-stage reaction.
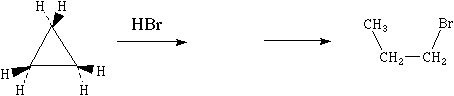
(A) Explain why cyclopropane contains an unusually high HOMO. [Hint: draw a picture that shows the angles between the sp3 hybrid AOs of carbon that overlap to form the C-C bonds in cyclopropane]
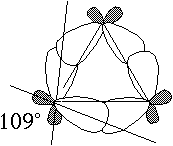 |
Although the C-C-C angles within cyclopropane must be 60°, the sp3 hybrids that should make the bonds among them make angles of 190° with one another. Since the hybrids on adjacent carbons do not point directly at one another, they do not overlap as well as they otherwise would, resulting in an unusually high s HOMO (and a lowish s* LUMO). [8 points]
|
(B) Name the LUMO of HBr with which cyclopropane's HOMO mixes in the first step of the reaction. What makes it a low LUMO?
(C) Use curved arrows to show both steps involved in the conversion of cyclopropane to n-propyl bromide.