|
|
|
 |
 |
 |
 |
|
|
|
Superficially the three types of halide R-X (where R = H, CH3, C2H5) undergo quite different types of reaction. The first is an acid that gives up its proton to a base. The second reacts with a "nucleophile" in an "SN2" reaction. The third gives SN2 as well, but it also undergoes "elimination" of HF to form a C=C double bond.
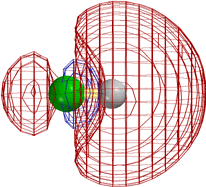
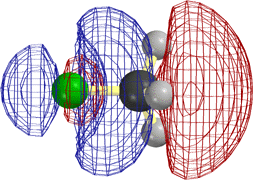
The following diagrams show that the different types of reactivity are conferred by closely analogous LUMOs (not necessarily the very lowest UMO, but close).
[Technical details: In the following calculations (MacSpartan ab initio with 6-31G(*) basis) the C2H5F orbital shown was actually LUMO+3, but it became LUMO+1 on stretching the terminal bonds by 0.3 Å. That is, it is the LUMO as reaction is occurring. Note that the red/blue color code is inverted in CH3F from that in the other three frames, but that this is insignificant, since one can arbitrarily change the sign everywhere in a wavefunction (multiply it by -1) and it is still the solution of the Schroedinger equation.]
|
|
|
 |
 |
 |
 |
|
|
|
In every case the vacant orbital is antibonding between a 2p σ AO of the green F and the adjacent atom (C or H). Occupancy of this orbital by electrons would cause the F-C (or F-H) bond to weaken, and F would leave as F-, taking along the previously bonding electron pair (not shown), of which it already had the lion's share,
In the first two cases the site for attack by the HOMO of the base or nucleophile is obviously the "back side" of the orbital on the atom attached to F. This is where it can make good overlap with this LUMO without running into other occupied orbitals.
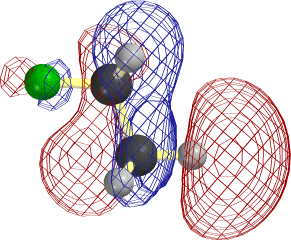
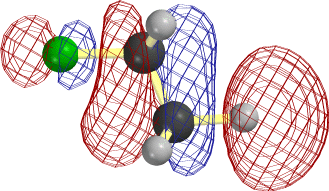
In the case of C2H5, it is easier to overlap the H atom from the lower right. Note that the LUMO is antibonding between C and this H, so that the new electrons cause this bond to rupture as well.
The frame at the lower right above, where the F-C and C-H bonds have begun to stretch, makes it clear that occupancy of this orbital is tantamount to forming the second, π, component of a double bond between the two C atoms.
|
Notice that the relevant MO of C2H5F can be thought of as the favorable (bonding) combination of two antibonding σ orbitals σ*FC and σ*CH. This favorable interaction survives in the form of a π-bond as F- and H+ break away. |
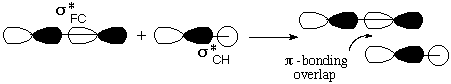 |
One can do bookkeeping of bonding electrons for the reactions above by using the curved-arrow formalism to show their shift. Note that the curved arrow originates where a moving electron pair is found in the starting material (on or between atoms) and ends where it is found in the product. B denotes a base or nucleophile, something with an unusually high HOMO.
|
|
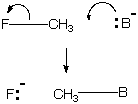 |
|
|
|
|
|
|